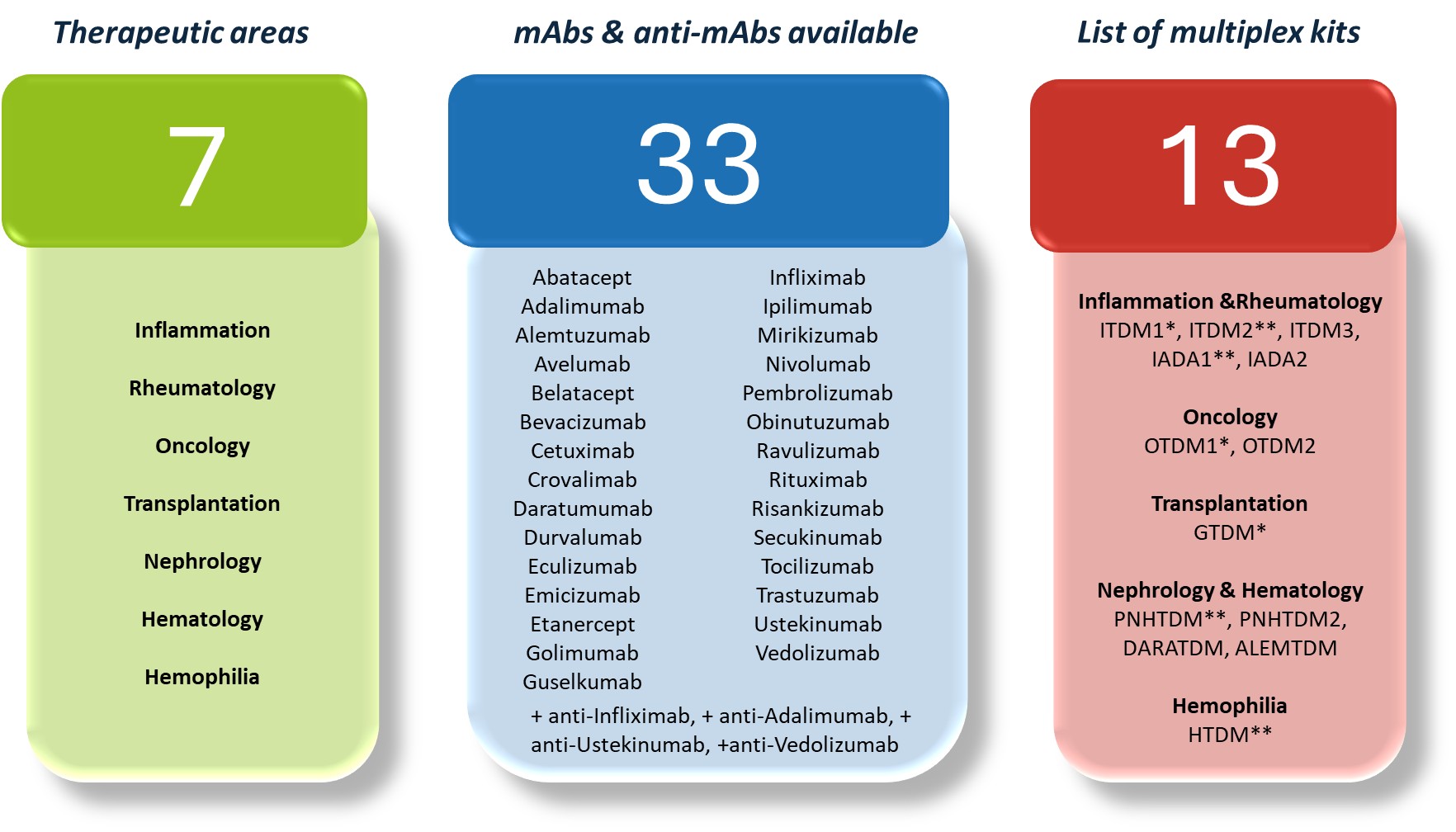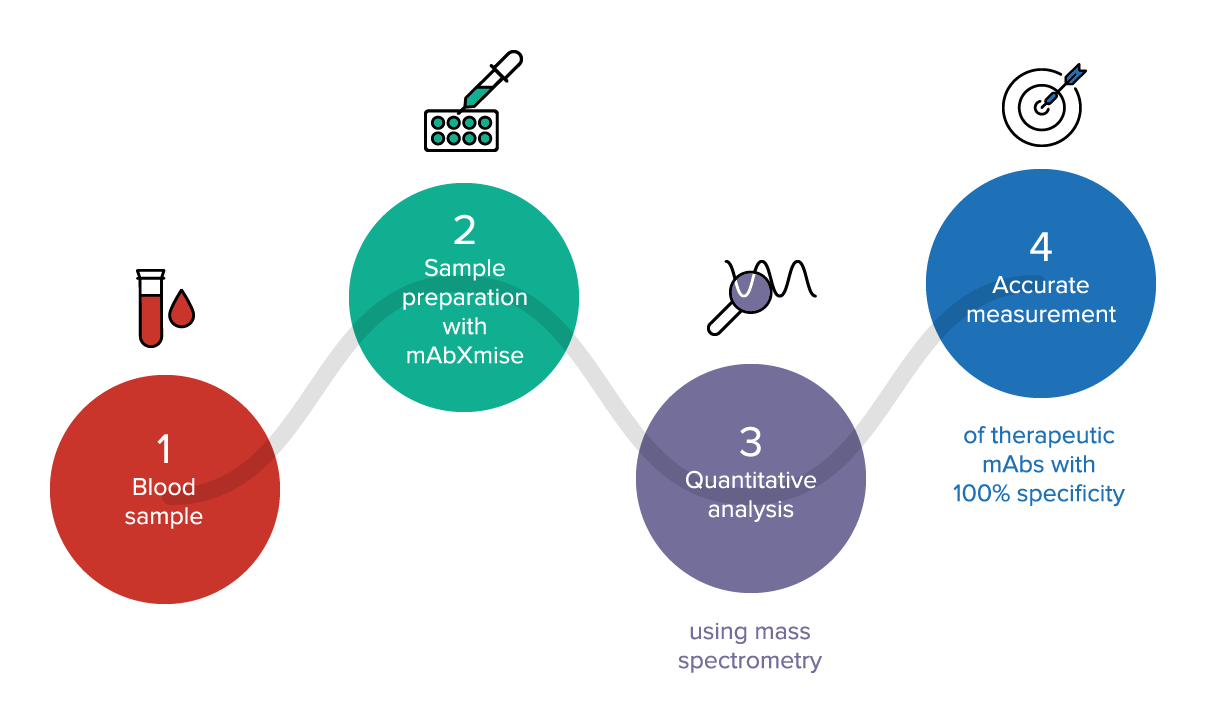
Webpages reserved for healthcare professionals based in EUROPE.
This website contains promotional content and information on medical devices, the use of which is reserved for healthcare professionals and laboratory professionals using IVD devices.
In application of the law of December 29,2011 relating to the strengthening of health security, I certify that I am a healthcare professional or a laboratory professional using IVD devices, I certify that I’m based in a European country and therefore I’m authorized to access this information.
For other countries, the webpages are under construction. Please contact us to get information contact@promise-proteomics.com